What is flexible batteries.....
Now a days with advancement in technology, there has developed may gadgets which are flexible and which can take user defined shape. These gadgets cannot run on its own as it require power source. This requirement of power source has made the gadgets limited in flexibility. It has made it necessary to develop a process to make batteries flexible too. Researches are being carried out to make the primary(non-rechargeable) as well as secondary(rechargeable) batteries flexible.
Basic Design
In general, a battery is made of one or several galvanic cells, where each cell consists of cathode, anode, separator, and in many cases current collectors. In flexible batteries all these components need to be flexible.
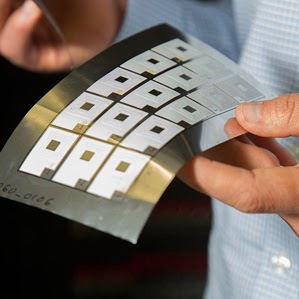
These batteries can be fabricated into different shapes and sizes and by different methods. One approach is to use polymer binders to fabricate composite electrodes where conductive additives are added to enhance their conductivity. The electrode materials can be printed or coated onto flexible substrates. The cells are assembled into flexible packaging materials to maintain bend-ability. Others approaches include the filtering of electrode suspension through filters to form free-standing films, or use flexible matrix to hold electrode materials. There are also other designs like cable batteries.
SPONSORED
Flexible, Printed Batteries
The MIT Technology Review reports that A California start up is developing flexible, rechargeable batteries that can be printed cheaply on commonly used industrial screen printers. Imprint Energy, of Alameda, California, has been testing its ultra thin zinc-polymer batteries in wrist-worn devices. The batteries probably won’t hold enough power to run a smartphone for days at a time, but it could enable small, light-weight wearable gadgets, medical devices, smart labels, and environmental sensors.
The batteries that power most laptops and smartphones contain lithium, which is highly reactive and has to be protected in ways that add size and bulk. While zinc is more stable, the water-based electrolytes in conventional zinc batteries cause zinc to form dendrites, branch-like structures that can grow from one electrode to the other, shorting the battery. Ho(company co founder Christine Ho ) developed a solid polymer electrolyte that avoids this problem, and also provides greater stability, and greater capacity for recharging.
Brooks Kincaid, the company’s co founder and president, says the batteries combine the best features of thin-film lithium batteries and printed batteries. Such thin-film batteries tend to be rechargeable, but they contain the reactive element, have limited capacity, and are expensive to manufacture. Printed batteries are non rechargeable, but they are cheap to make, typically use zinc, and offer higher capacity. The final application of such a printed secondary battery cell will either be a fully integrated part (e.g. printed on the same substrate as the functional – also printed circuitry) or in the form of a self adhesive label or sticker.
For more information on printed batteries and their fabrication.
Lithium polymer battery
A lithium polymer battery, or more correctly lithium-ion polymer battery (abbreviated variously as LiPo, LIP, Li-poly and others), is a rechargeable battery of lithium-ion technology in a pouch format.
Lithium-polymer differs from other battery systems in the type of electrolyte used. The original polymer design dating back to the 1970s uses a solid (dry) polymer electrolyte that resembles a plastic-like film. This insulator allows the exchange of ions (electrically charged atoms) and replaces the traditional porous separator that is soaked with electrolyte. A solid polymer has a poor conductivity at room temperature and the battery must be heated to 50–60°C (122–140°F) to enable current flow.The much anticipated “true plastic battery” promised in the early 2000s did not materialize; the conductivity could not be attained at ambient temperature.
To make the modern Li-polymer battery conductive at room temperature, gelled electrolyte is added. All Li-ion polymer cells today incorporate a micro porous separator with moisture. The correct term is “Lithium-ion polymer” (Li-ion polymer or Li-polymer for short). The gelled electrolyte becomes the catalyst that enhances the electrical conductivity. Li-polymer offers slightly higher specific energy and can be made thinner than conventional Li-ion, but the manufacturing cost increases by 10–30 percent. Despite the cost disadvantage, the market share of Li-polymer is growing.
Li-polymer cells also come in a flexible foil-type case (polymer laminate or pouch cell) that resembles a food package. While a standard Li-ion needs a rigid case to press the electrodes together, Li-polymer uses laminated sheets that do not need compression. A foil-type enclosure reduces the weight by more than 20 percent over the classic hard shell. Furthermore, thin film technology liberates the format design and the battery can be made into any shape, fitting neatly into stylish cell phones and laptops to make them smaller, thinner and lighter. Li-polymer can be made very slim to resemble a credit card.
Free-standing films approach
Because of their high energy and power density, lithium ion batteries that were mainly used for portable electronics are now extending to large applications such as power tools and vehicle electrification. Extensive research has been carried out to find new electrode materials and new electrode structure designs to improve energy densities for both anode and cathode.
To effectively increase the energy density on the device level, one needs to decrease the weight of each component. Previously, we have demonstrated that SiNWs( Silicon Nano Wires) on SS(Stainless Steel) can offer 10 times the capacity compared to commercial graphite. However, the weight of the metal current collector on the anode side is more than that of the active material; therefore, the improvement of the energy density on the anode side will be significantly compromised. Here we replace the heavy metal current collector of 10mg/cm2, with CNT(Carbon Nano Tube) film of 0.2 mg/cm2. Furthermore, the high capacity anode material, Si, was incorporated into such porous CNT films to form bi functional, freestanding films. Such CNT-Si films greatly improve 10 times the specific capacity of anodes even when the weight of current collector is considered. Such free-standing films successfully integrated the current collector and anode active material into a single sheet of film.We can also stack multiple layers of these CNT-Si films as anode and obtained high active material loading density per unit area. Two layers of this composite film with a total thickness of 8um will have an area capacity larger than 2 mAh/cm2, meeting the commercial standard.
Added advantage of free-standing CNT-Si film over pure sputtered-on Si film is ripples caused by repeated Si expansion and contraction during Li intercalation as it is clearly seen in the image. This ripples can relax the large strain in the film during Li cycling thus reduce the breaking of the film.
With the high capacity, low weight of the CNT-Si anode, further improvement of the energy density of the Li-ion battery will solely depend on the improvement of the cathode side.
Courtesy: Research work published in Department of Materials Science and Engineering, Stanford University, Stanford, California
Flexible cable batteries
LG Chem, a member of the LG conglomerate/chaebol and one of the largest chemical companies in the world, has devised a cable-type lithium-ion battery that’s just a few millimeters in diameter, and is flexible enough to be tied in knots, worn as a bracelet, or woven into textiles.
The underlying chemistry of the cable-type battery is the same as the lithium-ion battery in your smartphone or laptop — there’s an anode, a lithium cobalt oxide (LCO) cathode, an electrolyte — but instead of being laminated together in layers, they’re twisted into a hollow, flexible, spring-like helix.
LG Chem’s battery starts with thin strands of copper wire, which are coated with a nickel-tin (Ni-Sn) alloy to create the anode. These strands are twisted into a yarn, and then wrapped tightly around a 1.5mm-diameter rod. The rod is removed, leaving a strong spring. Next, aluminum wire is wrapped around the spring, and then the whole caboodle is dragged through a slurry of lithium cobalt oxide, which coats the aluminum wire and becomes the cathode. Finally, the anode-cathode spring is wrapped in a protective outer coating, and then an electrolyte is poured down the middle of the hollow spring to create a battery.
Now, flexible batteries as discussed above, they’ve all just standard, flat, laminated batteries made from sub-optimum materials, such as polymers. As such, as they have very low energy density, and they’re only bendy in the same way that a thin sheet of plastic is bendy . LG Chem’s cable-type batteries have the same voltage and energy density as your smartphone battery — but they’re thin and highly flexible to boot.
Instead of cylindrical batteries in laptops, or pouch-shaped batteries in smartphones and tablets, cable-type batteries would let you put batteries everywhere — around the outer edge of the chassis, around the screen’s bezel. Instead of creating devices with integrated batteries, you could instead wear a battery around your neck, or waist, or otherwise integrated into your clothing — and then plug into the device. Smartphones and tablets would instantly lose half their weight, and devices with flexible, roll-uppable displays would suddenly become feasible.
Courtesy: Research paper: DOI: 10.1002/adma.201202196
All these information has been collected from websites and battery manufactures across the world. Researchers are continually giving their efforts to develop new and innovative battery standard to pace up with the advancement of technology infield of flexible gadgets.
Now a days with advancement in technology, there has developed may gadgets which are flexible and which can take user defined shape. These gadgets cannot run on its own as it require power source. This requirement of power source has made the gadgets limited in flexibility. It has made it necessary to develop a process to make batteries flexible too. Researches are being carried out to make the primary(non-rechargeable) as well as secondary(rechargeable) batteries flexible.
Basic Design
In general, a battery is made of one or several galvanic cells, where each cell consists of cathode, anode, separator, and in many cases current collectors. In flexible batteries all these components need to be flexible.
These batteries can be fabricated into different shapes and sizes and by different methods. One approach is to use polymer binders to fabricate composite electrodes where conductive additives are added to enhance their conductivity. The electrode materials can be printed or coated onto flexible substrates. The cells are assembled into flexible packaging materials to maintain bend-ability. Others approaches include the filtering of electrode suspension through filters to form free-standing films, or use flexible matrix to hold electrode materials. There are also other designs like cable batteries.
SPONSORED
Flexible, Printed Batteries
The MIT Technology Review reports that A California start up is developing flexible, rechargeable batteries that can be printed cheaply on commonly used industrial screen printers. Imprint Energy, of Alameda, California, has been testing its ultra thin zinc-polymer batteries in wrist-worn devices. The batteries probably won’t hold enough power to run a smartphone for days at a time, but it could enable small, light-weight wearable gadgets, medical devices, smart labels, and environmental sensors.
The batteries that power most laptops and smartphones contain lithium, which is highly reactive and has to be protected in ways that add size and bulk. While zinc is more stable, the water-based electrolytes in conventional zinc batteries cause zinc to form dendrites, branch-like structures that can grow from one electrode to the other, shorting the battery. Ho(company co founder Christine Ho ) developed a solid polymer electrolyte that avoids this problem, and also provides greater stability, and greater capacity for recharging.
Brooks Kincaid, the company’s co founder and president, says the batteries combine the best features of thin-film lithium batteries and printed batteries. Such thin-film batteries tend to be rechargeable, but they contain the reactive element, have limited capacity, and are expensive to manufacture. Printed batteries are non rechargeable, but they are cheap to make, typically use zinc, and offer higher capacity. The final application of such a printed secondary battery cell will either be a fully integrated part (e.g. printed on the same substrate as the functional – also printed circuitry) or in the form of a self adhesive label or sticker.
For more information on printed batteries and their fabrication.
Lithium polymer battery
A lithium polymer battery, or more correctly lithium-ion polymer battery (abbreviated variously as LiPo, LIP, Li-poly and others), is a rechargeable battery of lithium-ion technology in a pouch format.
Lithium-polymer differs from other battery systems in the type of electrolyte used. The original polymer design dating back to the 1970s uses a solid (dry) polymer electrolyte that resembles a plastic-like film. This insulator allows the exchange of ions (electrically charged atoms) and replaces the traditional porous separator that is soaked with electrolyte. A solid polymer has a poor conductivity at room temperature and the battery must be heated to 50–60°C (122–140°F) to enable current flow.The much anticipated “true plastic battery” promised in the early 2000s did not materialize; the conductivity could not be attained at ambient temperature.
To make the modern Li-polymer battery conductive at room temperature, gelled electrolyte is added. All Li-ion polymer cells today incorporate a micro porous separator with moisture. The correct term is “Lithium-ion polymer” (Li-ion polymer or Li-polymer for short). The gelled electrolyte becomes the catalyst that enhances the electrical conductivity. Li-polymer offers slightly higher specific energy and can be made thinner than conventional Li-ion, but the manufacturing cost increases by 10–30 percent. Despite the cost disadvantage, the market share of Li-polymer is growing.
Li-polymer cells also come in a flexible foil-type case (polymer laminate or pouch cell) that resembles a food package. While a standard Li-ion needs a rigid case to press the electrodes together, Li-polymer uses laminated sheets that do not need compression. A foil-type enclosure reduces the weight by more than 20 percent over the classic hard shell. Furthermore, thin film technology liberates the format design and the battery can be made into any shape, fitting neatly into stylish cell phones and laptops to make them smaller, thinner and lighter. Li-polymer can be made very slim to resemble a credit card.
Free-standing films approach
Because of their high energy and power density, lithium ion batteries that were mainly used for portable electronics are now extending to large applications such as power tools and vehicle electrification. Extensive research has been carried out to find new electrode materials and new electrode structure designs to improve energy densities for both anode and cathode.
To effectively increase the energy density on the device level, one needs to decrease the weight of each component. Previously, we have demonstrated that SiNWs( Silicon Nano Wires) on SS(Stainless Steel) can offer 10 times the capacity compared to commercial graphite. However, the weight of the metal current collector on the anode side is more than that of the active material; therefore, the improvement of the energy density on the anode side will be significantly compromised. Here we replace the heavy metal current collector of 10mg/cm2, with CNT(Carbon Nano Tube) film of 0.2 mg/cm2. Furthermore, the high capacity anode material, Si, was incorporated into such porous CNT films to form bi functional, freestanding films. Such CNT-Si films greatly improve 10 times the specific capacity of anodes even when the weight of current collector is considered. Such free-standing films successfully integrated the current collector and anode active material into a single sheet of film.We can also stack multiple layers of these CNT-Si films as anode and obtained high active material loading density per unit area. Two layers of this composite film with a total thickness of 8um will have an area capacity larger than 2 mAh/cm2, meeting the commercial standard.
Added advantage of free-standing CNT-Si film over pure sputtered-on Si film is ripples caused by repeated Si expansion and contraction during Li intercalation as it is clearly seen in the image. This ripples can relax the large strain in the film during Li cycling thus reduce the breaking of the film.
With the high capacity, low weight of the CNT-Si anode, further improvement of the energy density of the Li-ion battery will solely depend on the improvement of the cathode side.
Courtesy: Research work published in Department of Materials Science and Engineering, Stanford University, Stanford, California
Flexible cable batteries
LG Chem, a member of the LG conglomerate/chaebol and one of the largest chemical companies in the world, has devised a cable-type lithium-ion battery that’s just a few millimeters in diameter, and is flexible enough to be tied in knots, worn as a bracelet, or woven into textiles.
The underlying chemistry of the cable-type battery is the same as the lithium-ion battery in your smartphone or laptop — there’s an anode, a lithium cobalt oxide (LCO) cathode, an electrolyte — but instead of being laminated together in layers, they’re twisted into a hollow, flexible, spring-like helix.
LG Chem’s battery starts with thin strands of copper wire, which are coated with a nickel-tin (Ni-Sn) alloy to create the anode. These strands are twisted into a yarn, and then wrapped tightly around a 1.5mm-diameter rod. The rod is removed, leaving a strong spring. Next, aluminum wire is wrapped around the spring, and then the whole caboodle is dragged through a slurry of lithium cobalt oxide, which coats the aluminum wire and becomes the cathode. Finally, the anode-cathode spring is wrapped in a protective outer coating, and then an electrolyte is poured down the middle of the hollow spring to create a battery.
Now, flexible batteries as discussed above, they’ve all just standard, flat, laminated batteries made from sub-optimum materials, such as polymers. As such, as they have very low energy density, and they’re only bendy in the same way that a thin sheet of plastic is bendy . LG Chem’s cable-type batteries have the same voltage and energy density as your smartphone battery — but they’re thin and highly flexible to boot.
Instead of cylindrical batteries in laptops, or pouch-shaped batteries in smartphones and tablets, cable-type batteries would let you put batteries everywhere — around the outer edge of the chassis, around the screen’s bezel. Instead of creating devices with integrated batteries, you could instead wear a battery around your neck, or waist, or otherwise integrated into your clothing — and then plug into the device. Smartphones and tablets would instantly lose half their weight, and devices with flexible, roll-uppable displays would suddenly become feasible.
Courtesy: Research paper: DOI: 10.1002/adma.201202196
All these information has been collected from websites and battery manufactures across the world. Researchers are continually giving their efforts to develop new and innovative battery standard to pace up with the advancement of technology infield of flexible gadgets.




No comments:
Post a Comment